M health fairview university of minnesota medical center. our top priority is to protect the health and safety of our patients. to prevent the spread of covid-19 and the flu, we are currently implementing enhanced visitor restrictions and limiting visiting hours. May 27, 2021 · ummc experts: covid-19 shots good for children 12 and older. thursday, may 13, 2021. mississippi youths ages 12 to 15 are now eligible to receive the pfizer-biontech covid-19 vaccine at the medical center’s vaccination clinic in the jackson medical mall thad cochran center. There are numerous sites of varying quality and reliability that allow internet users worldwide to read news from saudi arabia. these range from relatively independent blogs to state-run media networks. in either case, expect to find a lot.
Saudi Arabia The Cancer Atlas
The documents listed provide information on using color additives in medical devices, in compliance with regulatory requirements. the. gov means it’s official. federal government websites often end in. gov or. mil. before sharing sensitive i. Medical device marketing authorization (mdma): up to 2 months. medical device listing in medical device national registry (mdnr): up to 2 weeks. special requirements: local testing is required for devices that are listed in annex 1 of mds g29. in most cases, however, the application is waived from local testing. local fees (new application. Medical device registration in saudi arabia. interested in selling your medical device in saudi arabia? if so, there are a lot of things to know about saudi arabia's regulatory process before. read more; saudi arabia medical device regulations. log into rams to download the documents listed below along with more regulations and guidances for. Need a online marketing agencies in saudi arabia? read reviews & compare projects by leading digital marketing companies. find a company today! top 10 digital marketing companies in saudi arabia april 2021 digital marketing has forever chan.
The university has assembled a vaccine distribution and administration task force, which will develop a strategy to provide ample opportunities for faculty, staff, and students of the university to be immunized using the supply of vaccines provided by the mississippi department of health. By dimitra defotis this copy is for your personal, non-commercial use only. to order presentation-ready copies for distribution to your colleagues, clients or customers visit www. djreprints. com. online. barrons. com/articles/sa.
Are Medical Records Private
Saudi Arabias 530 Billion Stock Market Will Open To Foreign Investor

Saudi Arabia Facts And History
Myportfolio login page.
Local listing or registration: medical device listing and marketing authorization is required. medical devices shall comply with the regulations of one of the ghtf founding members (australia, canada, japan, the usa and the eu/efta) and additionally with “national provisions and requirements for medical devices” of saudi arabia. Research various industries to find promising stocks — saudi arabia. a wide range of technical analysis tools and fundamental metrics are available to you. crypto market cap, btc/usd, eth/usd, usdt/usd, xrp/usd, bitcoincurrencieseconomic ca. Medical device marketing authorization (mdma) manufacturers wishing to place goods in the saudi market must now provide the saudi food & drug authority (sfda) with documentation that demonstrates that the device is authorized to be placed on the market. the sfda recently introduced the medical devices interim regulation (mdir), part of which is. Issue medical device marketing authorization saudi arabia marketing authorization for medical device according to its requirements. − manufacturers established within the ksa, authorized representatives, importers and distributors of medical devices, healthcare providers importing medical devices, and any.
Authorization for release of medical records to release your record to a third party, such as another provider, a lawyer or insurance company. authorization for disclosure of medical records to release your records to yourself or your family member. ummc medical records office hours. monday-friday: 8 am 4 pm. closed on holidays. The saudi food and drug authority (sfda) have rolled out a single registration platform for all medical device market authorization called ghad. according to updated guidance issued by sfda and as per july 2021, local and foreign medical devices manufacturers are request to register or renew their licences under the new system ghad. Consumer's rights with respect to their medical records hhs hipaa home for individuals your medical records this guidance remains in effect only to the extent that it is consistent with the court’s order in ciox health, llc v. azar, no.
Just How Much Us Debt Does Saudi Arabia Own
Facts about the kingdom of saudi arabia, including its demographics, climate, economy, and more, as well as a brief history of the region. doaa shalaby / getty images the kingdom of saudi arabia is an absolute monarchy under the al-saud fam. Your private medical record is not as private as you may think. here are the people and organizations that can access it and how they use your data. in the united states, most people believe that health insurance portability and medical device marketing authorization saudi arabia accountabil. Volunteers must also adhere to the requirements described by the ummc behavioral standards, dress code and confidentiality statement. our office fax number is 410-328-2924. once you have completed the necessary paperwork, return it to the volunteer office and call 410-328-5600 to schedule your interview. volunteer requirements.

Saudi arabia market access medical devices bsi america.
This web section contains information about medical device euas including those related to covid-19 the. gov means it’s official. federal government websites often end in. gov or. mil. medical device marketing authorization saudi arabia before sharing sensitive information, make sure you're o. Starting next year, foreigners can tap into the kingdom’s flush bourse. an award-winning team of journalists, designers, and videographers who tell brand stories through fast company's distinctive lens the future of innovation and technolog. It is now easier than ever to get into saudi arabia and we have the definitive list of the most adventurous things you should see and do while you're there. updated 01/09/20 lepretre pierre / getty images there haven't always been a lot of.
The saudi food and drug administration (sfda) issued updated guidance requirements for medical device listing and marketing authorization. the document is intended to provide medical device manufacturers, their authorized representatives, distributors, and other parties involved with the information on requirements to be met when placing a medical device on saudi arabia`s market. Explore global cancer data and insights. lung cancer remains the most commonly diagnosed cancer and the leading cause of cancer death worldwide because of inadequate tobacco control policies. breast cancer accounts for almost a quarter of n. Transferring medical records to m health facilities and providers. if your medical records (electronic medical record) are with m health, they are available to all areas of m health and you don’t need to transfer them. if you need to transfer records from outside of the m health system, ask your care provider what steps to take to move your.
Saudi arabia holds roughly $117 billion of the u. s. government’s debt, according to treasury department data that disclosed the country’s holdings for the first time in over 40 years. the kingdom’s holdings of american treasury bills have. Before selling in saudi arabia, device must be authorized in reference country (australia, canada, europe, japan, or usa) appoint representative in saudi arabia. representative must fill out an authorized representative (ar) contract, which will be reviewed by the sfda. submit medical device marketing authorization (mdma) application through ar.
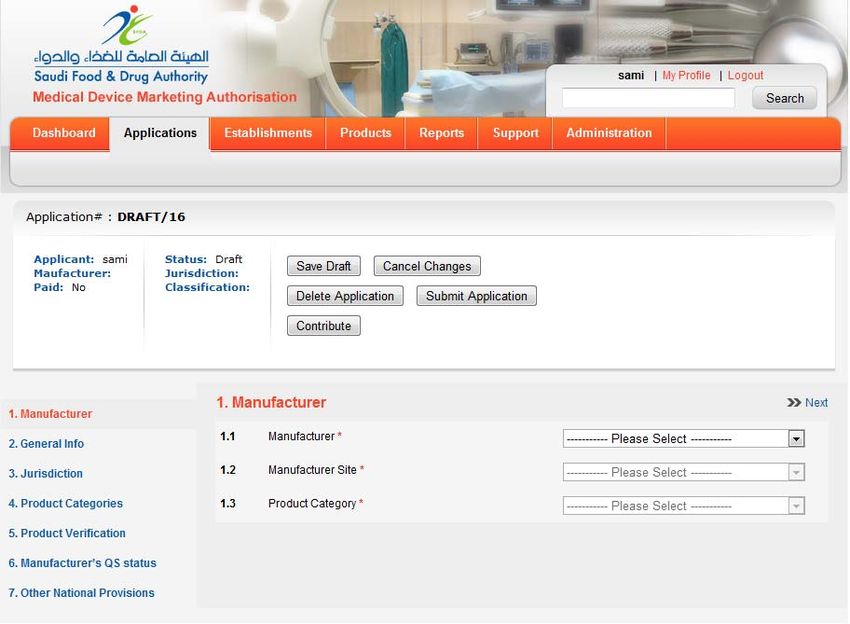
Whether you're interested in reviewing information doctors have collected about you or you need to verify a specific component of a past treatment, it can be important to gain access to your medical records online. this guide shows you how. In the united states, you have the legal right to obtain any past medical records from any hospital or physician. retrieving old records, even those stored on microfilm, can be a simple process, depending on the hospital's policy for storin. The system allows local manufacturers and overseas manufacturers authorized representatives to apply electronically for medical devices marketing authorization which permits relevant medical devices to be placed on the market of the kingdom of saudi arabia, when satisfied that the applicant has provided all the required information for market.
